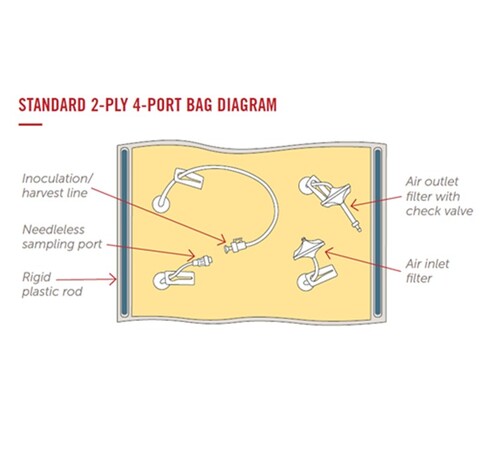
MOTION BIOREACTOR BAGS
Entegris Motion Bioreactor Bioprocess Bags are safe and secure single, double and triple barrier cell culture bags.
Starting with the preferred motion bioreactor, end users can choose the bag film, bag size, port geometry, port quantity, port location, tubing material, connector type, and more from Entegris’ extensive library of standard bioprocessing materials and components.
In addition, Entegris motion bioreactor bags are manufactured to the most exacting standards. To assure performance the customer can rely on, quality control inspections are performed from the raw material state to the final product. The entire process occurs under a quality system compliant to FDA standards.
Product Benefits
Customisable system design: provides design flexibility and allows the system to fit the process rather than the process adapt to the system
Consistently fast development and delivery minimize the risk of production delays
Personalised timely and useful attention ensures the solution meets your unique needs
Features
- Entegris motion bioreactor bags and associated components are sterilized using gamma irradiation between 25–40 kGy, unless requested otherwise
- Extractables and leachables (E&L) data available for all contact materials
- Full traceability for all materials
- Certificates of Analysis provided for each lot
- Entegris motion bioreactor bag assemblies are manufactured under a quality system designed to be compliant to 21 CFR Part 820: Quality. System Regulation
Application
- Mammalian and plant culture cell lines with extended mixing times